11 Which of the Following Is True About Bases
The pKa of HClO is 740 at 25C. The strong bases are excellent proton hydrogen ion acceptors and electron donors.

True Grit Conversions Easy How To S Magnetizing Arms Warhammer Paint Warhammer 40k Miniatures Painting Tutorial
They turn red litmus to blue.

. Arrange the following bases in order of increasing strength. Bases decrease the H in solution. All of the statements are true.
Question 11 Choose all the following statements that are. Bases donate hydrogen ions leftmathrmHright. Bases are sometimes called alkalis.
They have a sharp or sour taste. Which of the following statements about inheriting base class constructors is false. Bases are electrolytes.
Bases taste bitter feel slippery and neutralize acids. Acids and bases interact with each other in what is called a neutralization reaction. A HF does not dissolve in water.
The pH is neutralized to 7 if both the acid and base fully react. Which of the following statements is true. Problem 11 Medium Difficulty.
Bases are electron donor. Hence D - All the above. B Both bases require the same amount of HCl to reach pH 7 because they are both at the same initial pH.
Acids not bases can change the pH of a solution. C HF only partially dissociates into ions in water. Which of the following statements is true.
Describe and name acids and bases. This is a strong base. An Arrhenius acid produces H and an Arrhenius base produces OH-in aqueous solutions.
Acids mixed with bases make stronger bases. Two of the statements are true. B All Brønsted-Lowry acids are also Arrhenius acids.
Choose all that apply. E A Brønsted-Lowry base is a proton acceptor. Pick the base from the following.
A student measures the pH of a 00100M buffer solution made with HClO and NaClO as shown above. They release hydroxide ions in solution-is true about bases. Hence it is more available for donation.
Play this game to review Chemistry. D Arrhenius bases are ionic compounds in the pure state. Acids and bases dont react with each other.
Questions and Answers. D both B and C are correct. The strong bases can deprotonate weak acids.
If an inherited base-class constructor has default arguments the line of code in Part a causes the compiler to generate a derived-class constructor with the same default arguments. NH 3 PH 3 H 2 O and H 2 S. They tend to decrease the ph of a solution.
A The OH- ion is the basic species in the Brønsted-Lowry theory. On moving down the group with increase in atomic size the lone pair is diffused over larger volume and is less. Is concentrated over a small region.
Our experts have designed MCQ Questions for Class 11 Accountancy with Answers for all chapters in your NCERT Class 11 Accountancy book. In Chemistry A base is defined as a c hemical substance which when dissolve d in water gives a slippery feel it has a bitter taste and turns red litmus paper blue. Acids and bases can neutralize each other.
Bases are proton acceptors. Which of the following is correct. Bases react with acids to produce a salt and water.
The Calvin cycle of photosynthesis begins when A. View solution. Properties of the Strong Bases.
C The weak base will require less HCl to bring the pH to 7 than the strong base. Another definition is that a base is referred to as a species that gives out hydroxide OH- ions in aqueous solution it also freely donates electrons and takes protons. However its never a good idea to touch a solution to test it because these bases tend to be caustic.
Acids mixed with bases neutralize each other. Acids mixed with bases make stronger acids. An aqueous solution of this base would be acidic.
The following Theory Base of Accounting Class 11 Accountancy MCQ Questions has been designed based on the latest syllabus and examination pattern for Class 11. Which of the following statements is true concerning acids and bases. They release hydroxide ions in solution.
This base ionizes slightly in aqueous solution. Which of the following statements is true concerning acids and bases. Which of the following qualifies as a Brønsted-Lowry base.
Correct option is D Among the hydrides of group 15 ammonia NH 3. D both B and C are correct. A HCl will not bring the pH of either solution to pH 7.
Due to smaller atomic size the lone pair electrons on N in NH 3. Acids and bases dont react with each other. Acids and bases cannot mix together.
D The strong base will require less HCl to bring the pH to 7 than the weak base. Bases have a distinctly bitter taste. They tend to decrease the pH of a solution.
H 2 S H 2 O PH 3 NH 3. Acids mixed with bases make stronger bases. A K 2 SO 3 is a stronger base than KHSO 3 b K 2 CO 3 is a weaker base than KHCO 3 c NaHSO 3 is a stronger acid than NaHSO 4.
This is a concentrated base. Which of the following statements isare true of bases. Acids taste sour may sting and neutralize bases.
Acids mixed with bases neutralize each other. 11option AB are correct Because the ph of the stomach is about 15 to 2 This environment is required for the activation of pepsin This is brought abou View the full answer Transcribed image text. The products of the reaction are a salt and water.
B HF has a weak conjugate base F-. They have a sharp or sour taste. Was this answer helpful.
Acids donate hydroxide ions leftmathrmOH-right. They release hydroxide ions in solution. 1 on a question Which of the following is true about bases.
Acids mixed with bases make stronger acids. C All Brønsted-Lowry bases are also Arrhenius bases. View solution Which of the following is not a base.
Bases have more hydroxide ions that than hydronium ions. Which of the following is true about bases. A base has a Kb of 25 x 1011.
Chapter 11 Acids and Bases Practice Problems Section 111 Acids and Bases Goal. Aqueous solutions of strong bases are slippery and soapy. They help to build up cells.
Based on this information which of the following best compares the relative concentrations of ClO and HClO in the buffer solution.

Acids And Bases I Introduction

11 Methods For Applying Mesh To Create A Wreath Base How To Make A Wreath Youtube Deco Mesh Wreaths Diy Deco Mesh Wreaths Tutorials Making Mesh Wreaths

How To Attract The Man Of Your Dreams Love Note In 2021 Original Quotes Emotional Connection Finding True Love

Surprisingly Healthy Smoothie Recipe Green Smoothie Bowl Green Smoothie Easy Green Smoothie

White Women 8 5 Vans Van Design White Sneaker

Acids Bases Ph Scale Seek And Find Science Doodle Printable And Digital Science Doodles Science Lessons Elementary Teaching Middle School Science

Snow White Inspired Wedding Stationery Snow White Wedding Wedding Stationery Disney Wedding

Town Square Series Decorative Pole Base Ceiling Lights Light Pole Towns

Acids And Bases Ck 12 Foundation

Cheerleading A Reading Comprehension Worksheet Free Esl Printable Worksheets Mad Reading Comprehension Reading Comprehension Texts Comprehension Worksheets

Pin On Relationships Boundries Love
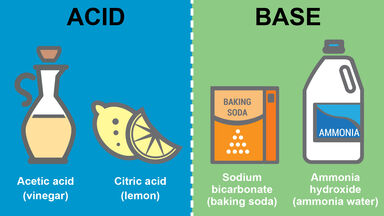
Difference Between Acids And Bases Key Properties

11in Diameter Round Black Marble Lamp Base Bam11b Grand Brass Lamp Parts Llc Marble Lamp Base Marble Lamp Lamp Parts

Pin By Clutz On Small Collectables Tiffany Lamps Small Collectibles Lamp Bases




Comments
Post a Comment